Table of Contents
Ans. (b) 45.3
Explanation:
The molecular mass of sodium carbonate is 106.
(2Na - 46, 1C = 12, and 3O = 48)
since atomic mass of 3 oxygen atoms is 48
\text{Therefore 100 }× \dfrac{48}{106}
= 45.3%
Ans. (C)\space C_6H_{12}O_6
Explanation:
molar mass of carbon= 12.
molar mass of hydrogen=1.
molar mass of =16.
mass of CH_2O=12+2(1)+16=30.\\
molecular wieght of compound given is 180.
so the molecular wieght is
\dfrac{180}{30} = 6.\\
⟹ molecular formula cound is C_6H_{12}O_6
Explanation:
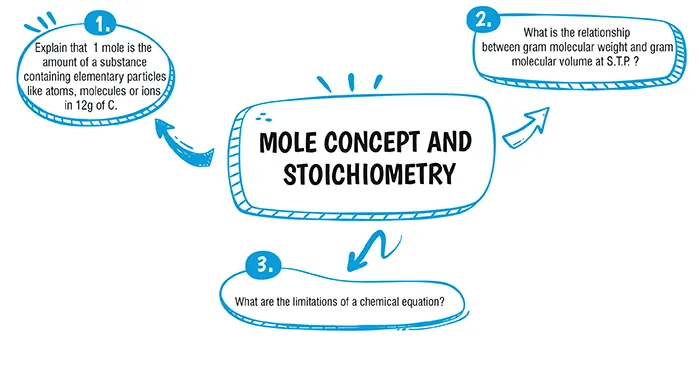
A mole is the quantity of a substance that contains the same number of particles as present in 12g of Carbon. This is the definition of mole. Here carbon-12 isotope is used as the reference standard because this is the most abundant carbon isotope found on earth. The further extension of this concept of mole is Avogadro's law.
Explanation:
The density of a gas is defined as mass per unit volume. Volume is usually taken as 1 dm^3 at S.T.P.Molar volume of hydrogen: Density of hydrogen = 0 09 gm/dm^3 at S.T.P. Gram molecular weight of hydrogen = 2.016 g 0.09 g of hydrogen occupies volume = 1 dm^3 at S.T.P. 2.016 g of hydrogen occupies volume = dm^3 at S.T.P. = 22.4 dm^3 at S.T.P. As 2.016 g of hydrogen = 1 gram molecular weight,
∴ 1 gram molecular weight of hydrogen occupies 22.4 dm^3 at S.T.P.
Explanation:
The following are the limitations of the chemical equation:
1. It doesn’t tell us about the physical state of reactants such as solid, liquid, or gaseous.
2. We can’t predict whether an equation is reversible or irreversible.
3. Actual concentration and dilution are unknown.
4. The time duration required for a particular chemical reaction is not known.
5. Whether the reaction goes to completion or is stopped in between i.e., the extent to which a reaction takes place is not known from a chemical equation
6. The parameters that affect a chemical reaction such as temperature, pressure, catalyst, etc are unknown.
Download Mind Map of this chapter
Download NowWant to Practice Mock Tests of this chapter
Practice NowDownload Important Questions of this chapter
Download Now| Chapter No. | Chapter Name |
|---|---|
| Chapter 1 | Periodic Properties and Variations of Properties |
| Chapter 2 | Chemical Bonding |
| Chapter 3 | Study of Acids, Bases and Salts |
| Chapter 4 | Analytical Chemistry |
| Chapter 5 | Mole concept and Stoichiometry |
| Chapter 6 | Electrolysis |
| Chapter 7 | Metallurgy |
| Chapter 8 | Study of Compounds : Hydrogen Chloride |
| Chapter 9 | Study of Compounds : Ammonia and Nitric Acid |
| Chapter 10 | Study of Compounds : Sulphuric Acid |
| Chapter 11 | Organic Compounds |
| Chapter Wise Important Questions for ICSE Board Class 10 Chemistry |
|---|
| Periodic Properties and Variations of Properties |
| Chemical Bonding |
| Study of Acids, Bases and Salts |
| Analytical Chemistry |
| Mole concept and Stoichiometry |
| Electrolysis |
| Metallurgy |
| Study of Compounds : Hydrogen Chloride |
| Study of Compounds : Ammonia and Nitric Acid |
| Study of Compounds : Sulphuric Acid |
| Organic Compounds |
CBSE Important Questions Class 10
ICSE Important Questions Class 10
CBSE Important Questions Class 10
ICSE Important Questions Class 10