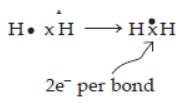Here, you’ll discover significant inquiries pertaining to Chapter 2: Chemical Bonding for ICSE Class 10 Chemistry. These inquiries are carefully designed to aid students in preparing for the ICSE Class 10 Chemistry Examination in 2024–25. Engaging with different question formats allows students to address uncertainties, improve their exam preparedness, boost their self-assurance, and polish their ability to solve problems.
In Class 10 Chemical Bonding, you will delve into the Electron Dot Structures of Electrovalent compounds like NaCl, MgCl_2, and CaO. You’ll also explore the distinctive properties of electrovalent compounds, including their physical states, melting and boiling points, conductivity (both heat and electricity), dissociation in solutions, and when in a molten state, which will be connected to electrolysis. Moreover, the chapter covers the Electron Dot Structures of covalent molecules based on the concepts of duplet and octet of electrons. Examples include hydrogen, oxygen, chlorine, nitrogen, ammonia, carbon tetrachloride, and methane. It will also discuss Polar Covalent compounds, which are determined by differences in electronegativity. Examples provided are HCl, NH_3, and H_2O, along with their respective structures. Furthermore, you’ll learn about the characteristic properties of covalent compounds, such as their physical states, melting and boiling points, conductivity (heat and electricity), and ionisation in solution. A comparison between Electrovalent and Covalent compounds will be made. The chapter will define Coordinate Bonding and explain the lone pair effect observed in the oxygen atom of the water molecule and the nitrogen atom of the ammonia molecule. These explanations will help illustrate the formation of H3O^+ and OH^- ions in water and the NH_4^+ ion. The concept of a lone pair will be clarified, and the formation of hydronium ions and ammonium ions will be elucidated using electron dot diagrams. If you’re looking for chemical bonding class 10 ICSE important questions or questions on chemical bonding class 10, make sure to refer to the Oswal textbooks or oswal.io for comprehensive practice.
In ICSE Class 10 Chapter 2, “Chemical Bonding,” the concept of chemical bonding comes to the forefront. This fundamental process involves the establishment of chemical connections among two or more atoms, molecules, or ions, ultimately culminating in the formation of a chemical compound. These chemical bonds play a pivotal role in maintaining the unity of the constituent elements within the resulting compound. The driving force behind this cohesion, which brings various components, such as atoms and ions, together and stabilises them by reducing the overall energy, is aptly termed chemical bonding. As we delve deeper into this topic, it becomes evident that the strength of these chemical bonds among constituents significantly influences the stability of the resulting compound. Stronger bonds contribute to greater stability, ensuring the compound’s durability. Conversely, weak chemical bonding between constituents results in diminished stability, rendering the compound susceptible to further reactions aimed at producing more stable compounds with stronger bonds. In their pursuit of stability, atoms strive to minimise their energy levels. In interactions between different forms of matter, forces come into play. When these forces are attractive, they lead to an energy reduction. Conversely, repulsive forces drive an increase in energy. The attractive force responsible for binding two atoms together is recognized as a chemical bond. Thus, the study of chemical bonding forms the cornerstone of understanding the intricate relationships between atoms, molecules, and ions, influencing the stability and reactivity of compounds. For questions on chemical bonding class 10 ICSE important questions, you should refer to your class materials and textbooks for a comprehensive set of practice questions and exercises.
The exploration of “Chemical Bonding” in ICSE Class 10 Chemistry has provided a fundamental understanding of the forces that bind atoms, molecules, and ions together to form chemical compounds. The study of chemical bonds, both strong and weak, is paramount in comprehending the stability and reactivity of these compounds.Throughout this chapter, we’ve delved into the intricacies of attractive and repulsive forces that govern the behaviour of matter at the atomic and molecular levels. For those seeking to excel in this critical area of chemistry, additional practice resources can be invaluable. oswal.io offers a comprehensive collection of questions and study materials tailored to enhance your learning experience.