Table of Contents
Ans. (d)
Explanation:
Alkanes are known as saturated compounds. All the alkenes and alkynes with double and triple bonds in their carbon atoms are known as unsaturated alkenes. Among the given options, propene is an alkene with the formula C_3H_6. It contains a double bond between two carbon atoms, making it unsaturated. Propyne is an alkyne with the formula C_3H_4. It contains a triple bond between two carbon atoms, making it unsaturated.
Ans. (c)
Explanation:
The atomic mass unit (amu) by which successive members of a homologous series of hydrocarbons vary is based on the addition of a CH_2 group.
Each successive member of a homologous series of alkanes, for example, differs by one CH_2 group, which corresponds to an increase of 14 amu (12 amu for the carbon and 2 amu for the hydrogen).
Explanation:
The molecular formula of two consecutive members of a homologous series differ by CH_2.
Explanation:
Catenation and tetravalency are the two properties of carbon which leads to the formation of a large number of carbon compounds.
Explanation:
(i) When ethanol (C₂H₅OH) is heated at 443 K in the presence of concentrated sulfuric acid (H₂SO₄), it undergoes a dehydration reaction to form ethene (C₂H₄), also known as ethylene. The reaction can be represented as:
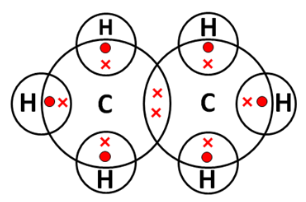
\\C_2H_5OH \underrightarrow{\space \space 443 \text{K, conc. H}_2\text{SO}_4 \space \space } C_2 H_4 + H_2O \\[5 bp] Electron Dot Structure of Ethene
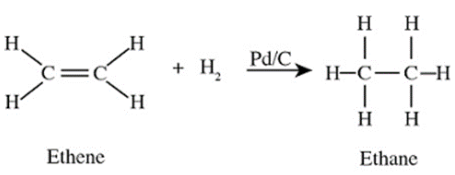
(ii) Hydrogenation is a chemical reaction in which hydrogen (H₂) is added to an unsaturated hydrocarbon, resulting in the saturation of the compound. This process generally involves the addition of hydrogen to double or triple bonds in alkenes or alkynes, converting them into alkanes. For example, ethene (C₂H₄), which has a double bond, reacts with hydrogen gas (H₂) to form ethane (C₂H₆), a saturated hydrocarbon.
The industrial applications of hydrogenation are as follows:
· Converts liquid vegetable oils into vegetable ghee.
· Removes sulphur and nitrogen compounds from crude oil.
· Used in the synthesis of various pharmaceutical compounds.
| Chapter No. | Chapter Name |
|---|---|
| Chapter 1 | Chemical Reactions and Equations |
| Chapter 2 | Acid, Bases and Salts |
| Chapter 3 | Metals and Non-Metals |
| Chapter 4 | Carbon and its Compounds |
| Chapter 5 | Life Processes |
| Chapter 6 | Control and Coordination |
| Chapter 7 | How do Organisms Reproduce |
| Chapter 8 | Heredity |
| Chapter 9 | Light : Reflection and Refraction |
| Chapter 10 | The Human Eye and the Colourful world |
| Chapter 11 | Electricity |
| Chapter 12 | Magnetic Effects of Electric Current |
| Chapter 13 | Our Environment |

| Chapter Wise Important Questions for CBSE Board Class 10 Science |
|---|
| Chemical Reactions and Equations |
| Acid, Bases and Salts |
| Metals and Non-Metals |
| Carbon and its Compounds |
| Life Processes |
| Control and Coordination |
| How do Organisms Reproduce |
| Heredity |
| Light : Reflection and Refraction |
| The Human Eye and the Colourful world |
| Electricity |
| Magnetic Effects of Electric Current |
| Our Environment |
CBSE Important Questions Class 10
ICSE Important Questions Class 10
CBSE Important Questions Class 10
ICSE Important Questions Class 10