Table of Contents
Ans. (c)
Explanation:
During the preparation of hydrogen chloride gas, especially on a humid day, the gas may carry moisture due to the presence of water vapour in the air. Calcium chloride (CaCl₂) is a drying agent, and its role in the guard tube is to absorb any moisture from the hydrogen chloride gas, ensuring that the gas remains dry.
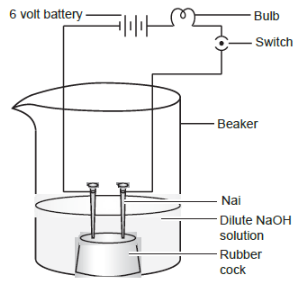
Ans. (c)
Explanation:
Statement (ii) is correct because NaOH is a strong base that dissociates completely in water to furnish ions (Na⁺ and OH⁻), which can conduct electricity, causing the bulb to glow.
Explanation:
The general reaction of an acid with a metal carbonate produces salt, carbon dioxide (CO₂), and water (H₂O). The balanced chemical equation for this reaction is:
Acid + Metal carbonate → Salt + CO_2 + H_2O \\[5 bp]
For example, when hydrochloric acid (HCl) reacts with calcium carbonate (CaCO₃), the balanced chemical equation is:
2HCl(aq) + CaCO_3(s) → CaCl_2(aq) +CO_2(g) + H_2O(l)
Explanation:
When iron reacts with steam, it forms iron(III) oxide (Fe₃O₄) and hydrogen gas (H₂). The reaction takes place at high temperatures. The balanced chemical equation is:
The compound of iron obtained is iron(III) oxide (Fe₃O₄).
Explanation:
The substance is likely copper(II) sulphate pentahydrate (CuSO₄·5H₂O).
Explanation of the Phenomenon:
CuSO_4⋅5H_2O(s)\underrightarrow{\space \space \text{HEAT}\space \space } CuSO_4(s) + 5H_2O(g) \\[5 bp]
CuSO_4(s) + 5H_2O(l) → CuSO_4⋅5H_2O(s)
| Chapter No. | Chapter Name |
|---|---|
| Chapter 1 | Chemical Reactions and Equations |
| Chapter 2 | Acid, Bases and Salts |
| Chapter 3 | Metals and Non-Metals |
| Chapter 4 | Carbon and its Compounds |
| Chapter 5 | Life Processes |
| Chapter 6 | Control and Coordination |
| Chapter 7 | How do Organisms Reproduce |
| Chapter 8 | Heredity |
| Chapter 9 | Light : Reflection and Refraction |
| Chapter 10 | The Human Eye and the Colourful world |
| Chapter 11 | Electricity |
| Chapter 12 | Magnetic Effects of Electric Current |
| Chapter 13 | Our Environment |
| Chapter Wise Important Questions for CBSE Board Class 10 Science |
|---|
| Chemical Reactions and Equations |
| Acid, Bases and Salts |
| Metals and Non-Metals |
| Carbon and its Compounds |
| Life Processes |
| Control and Coordination |
| How do Organisms Reproduce |
| Heredity |
| Light : Reflection and Refraction |
| The Human Eye and the Colourful world |
| Electricity |
| Magnetic Effects of Electric Current |
| Our Environment |
CBSE Important Questions Class 10
ICSE Important Questions Class 10
CBSE Important Questions Class 10
ICSE Important Questions Class 10