Table of Contents
Ans. (a) Water can extract more heat without raising its temperature significantly.
Explanation:
When water passes through a pipe surrounded by a hot part of the engine, heat energy is removed from those parts. Because water has high specific heat capacity, water in pipes may extract more heat from the environment without significantly raising its temperature. As a result, the car’s radiator and generator are both filled with water.
Ans. (c) The direction of current flow
Explanation:
Regardless of the direction of current flow, the amount of heat produced in the transmission wire (in general, a current carrying conductor) is determined by the amount of current flowing through it and the resistance of the conductor.
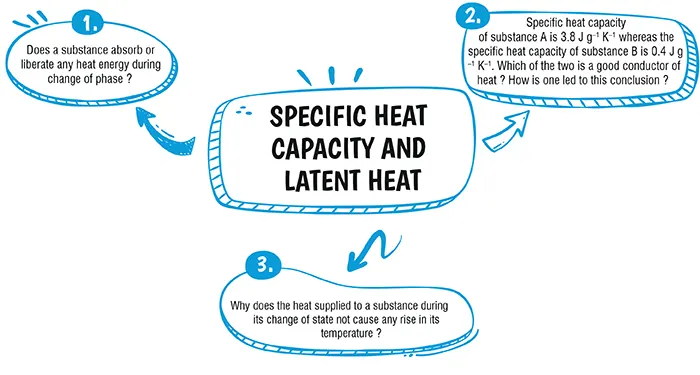
Explanation:
Yes, heat is either absorbed or released during change of phase. Phase change, also known as a phase transition, refers to the transformation of matter from one state (or phase) to another, such as from solid to liquid or liquid to gas. These transitions require or release energy, and this energy is typically in the form of heat. The heat associated with a phase change is called latent heat.
Explanation:
‘B’ is a good conductor of heat, because for the same heat energy and same mass, the rise in temperature of B will be more. Hence, ‘B’ is a good conductor of heat.
Explanation:
During the state change heat supplied increases potential energy of molecules. As distance between molecules increases, work is done by heat supplied against attractive force.
Download Mind Map of this chapter
Download NowWant to Practice Mock Tests of this chapter
Practice NowDownload Important Questions of this chapter
Download Now| Chapter No. | Chapter Name |
|---|---|
| Chapter 1 | Force Work Power and Energy |
| Chapter 2 | Simple Machines |
| Chapter 3 | Refraction of Light |
| Chapter 4 | Refraction Through Lenses |
| Chapter 5 | Spectrum |
| Chapter 6 | Sound |
| Chapter 7 | Electricity |
| Chapter 8 | Electrical Power and Household Circuits |
| Chapter 11 | Magnetic Effect of Current |
| Chapter 12 | Specific Heat Capacity and Latent Heat |
| Chapter 13 | Radioactivity and Nuclear Energy |
| Chapter Wise Important Questions for ICSE Board Class 10 Physics |
|---|
| Force Work Power and Energy |
| Simple Machines |
| Refraction of Light |
| Refraction Through Lenses |
| Spectrum |
| Sound |
| Electricity |
| Electrical Power and Household Circuits |
| Magnetic Effect of Current |
| Specific Heat Capacity and Latent Heat |
| Radioactivity and Nuclear Energy |
CBSE Important Questions Class 10
ICSE Important Questions Class 10
CBSE Important Questions Class 10
ICSE Important Questions Class 10